Acute T-cell lymphoblastic leukemia (T-ALL) is an aggressive hematologic malignancy in children and young adults. When compared to B-ALL, T-ALL has a higher rate of induction failure as well as relapse, with very poor overall survival in both scenarios. Furthermore, T-ALL has not benefitted from immune-mediated therapies and continues to pose an ongoing treatment challenge with an unmet therapeutic need.
DHODH has been highlighted as a potential therapeutic target in many hematologic and solid tumor malignancies including AML, neuroblastoma, glioblastoma, pancreatic cancer, and breast cancer. DHODH inhibition has a robust anti-leukemia effect in both in vitro and in vivo models of T-ALL. We have confirmed this sensitivity in vitro as well as in vivo in three murine models of T-ALL, including an aggressive NOTCH driven model in syngeneic recipients and two human patient derived xenografts.
Given the goal of making DHODHi therapy available to young children with relapsed/refractory T-ALL, we wished to understand the ramifications of nucleotide starvation on normal T-cells and other hematopoietic progenitors. To specifically address this, we turned our attention to the effects of DHODH inhibition on the developing thymus. This compartment would be of particular interest in pediatric patients, whose thymi have not yet finished developing.
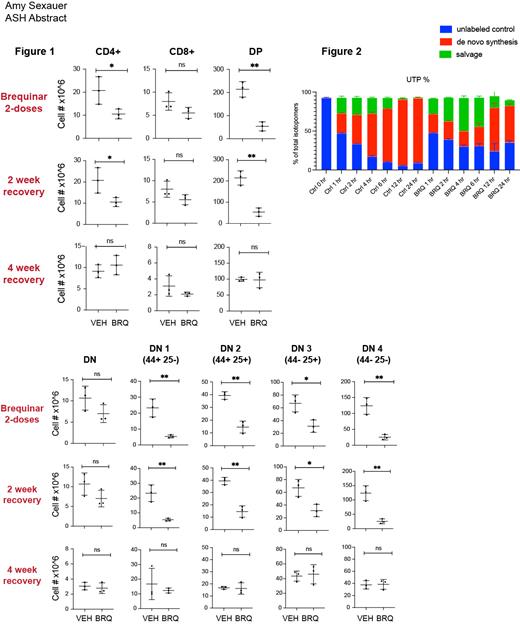
We studied juvenile mice (3 weeks) whose thymi are just reaching their peak size and growth rate. These mice were treated with the small molecule DHODHi brequinar (BRQ) 50 mg/kg every 72 hours, and were paired with age and gender-matched controls at each time point of analysis. A subset of mice were analyzed following two doses of BRQ, and thymus, bone marrow, spleen, and peripheral blood were analyzed for T-cell populations, both relative and absolute numbers. Another cohort was allowed to “recover” for 2 weeks following treatment before analysis, and a third cohort was allowed a four week recovery period. We discovered that initial treatment with brequinar significantly impacts T-cells across all stages of development ( Figure 1). This is expected, as developing T-cells are rapidly proliferative (often dividing as quickly as every 6 hours) and therefore highly dependent on their supply of nucleotides for DNA replication as well as transcription. However, after the two-week and the four-week recovery periods, these differences are no longer detectable, demonstrating the resilience of the developing thymus, and demonstrating that the effects of nucleotide starvation are temporary and reversible. These data are encouraging, as this may also suggest that the developing human thymus which is similarly rapidly proliferative in nature would also recover once nucleotide starvation was removed.
Based on these data, we were interested in understanding the differential sensitivity of normal thymocytes as compared to malignant T-lymphoblasts to DHODHi. The resilience shown by normal thymocytes following treatment with BRQ may be due to their ability to tolerate periods of inhibition of de novo pyrimidine synthesis by preferentially relying on extracellular nucleotide salvage, whereas the sensitivity of malignant T-cells to DHODH may be due to their lack of ability to utilize the salvage pathway.
We performed experiments to understand how cells balance de novo synthesis vs extracellular salvage. First, we performed metabolite flux tracing in Jurkat cells to directly measure nucleotide salvage. Here, we followed incorporation of isotopically labeled glutamine ( 15N) and uridine ( 13C) to determine the relative contributions of synthesis vs salvage, as de novo pyrimidine synthesis initiates from glutamine and aspartate while a cell can also use membrane nucleoside transporters (SLC28A and SLC29A family of proteins) to salvage extracellular uridine. These experiments were performed in the presence and absence of BRQ. Once BRQ is added and the cell's ability to synthesize pyrimidines is shut down, the cell must rely more on extracellular salvage as shown in Figure 2. A defective or less efficient process of extracellular salvage could help to explain this cell line's unusual dependence on de novo nucleotide synthesis.
Overall, we hope that these findings will pave the way for additional preclinical studies and eventually clinical trials for pediatric patients with T-cell disease.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal